Surgical instruments are a costly investment to any hospital. The care and maintenance of these instruments is critical to their performance during surgery, as well as patient care.
The Sterile Processing Department (Central Supply, or Sterile Processing Service as it is also known), is the service within the hospital in which medical/surgical supplies and equipment, both sterile and non-sterile, are cleaned, prepared, processed, stored, and issued for patient care.
Sterile Processing Departments are typically divided into four major areas to accomplish the functions of decontamination; assembly and sterile processing, sterile storage and distribution.
In the decontamination area, reusable equipment, instruments, and supplies are cleaned and decontaminated by means of manual or mechanical cleaning processes and chemical disinfection. Following the cleaning process the microscopic inspection of the instruments takes place.
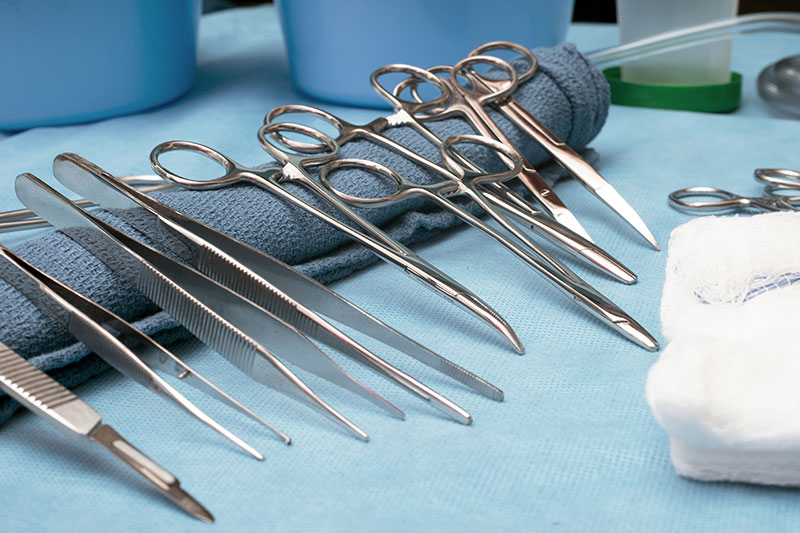
Visual inspection for quality control
After cleaning, all instruments should undergo inspection before being packaged for reuse or storage. The importance of inspecting each instrument cannot be overemphasized. A visual check for cleanliness and dryness should be made for all items washed, as part of the decontamination process, before being packaged for reuse or storage.
The condition of the instrument has a significant effect on how adequately it can be cleaned. Instruments subjected to rough handling will develop scratches and roughened surfaces over time, which will trap and hold dirt. Damaged surfaces not only allow dirt and bacteria to collect, but can also be potentially dangerous for both medical staff and patients.
Box locks, serrations, and crevices should be critically inspected for cleanliness. Instruments with cutting edges such as scissors, rongeurs, chisels, curettes, etc., should be checked for sharpness. There should be no dull spots, chips, or dents. Hinged instruments such as clamps and forceps should be checked for stiffness and alignment of jaws and teeth. Tips should be properly aligned, jaws should meet perfectly, and joints should move easily.
Ratchets should close easily and hold firmly. Any instruments with pins or screws should be inspected to make sure they are intact. Plated instruments should be checked to make sure there are no chips, worn spots, or sharp edges. Worn spots can rust during autoclaving. Chipped plating can trap dirt and can also damage tissue and rubber gloves when used, if not cleaned properly.
If any problems are noticed during the inspection process, these instruments should be either cleaned again, or sent for repair depending on the problem observed.
“There are a number of visualization tools on the market for visual inspection of instrumentation. However, stereo inspection microscopes provide technicians with a 3D view of instruments and the ability to view all sides of the instrument at once, clearly and effectively. Ideal for visualizing troubled areas like box locks and jaws, which often harbor residual bioburden.”
A general guide on how the instruments are inspected
Forceps and Hemostats: A visual test would be to close the jaws lightly. If they overlap they are out of alignment and need re-aligning. If the forceps have serrated jaws they should be checked to see that they mesh fully. There should be no play in the box joint of the instrument.
Scissors: Blades should be checked for burrs and they should be in good approximation all the way down the length. There should not be excessive fretting around the pivot pin which would lead to possible corrosion and breakage.
Needle Holders: A needle should be clamped in the jaw and the instrument closed to ensure that the needle can not be moved or turned easily.

Suggested stereo inspection system configuration
Lynx EVO eyepiece-less stereo microscope: Multi-Axis or Ergo stand, LED ring-light and magnification ranging from 6x – 90x.
Tracking and traceability of surgical instruments
It is important to be able to trace surgical instruments through the decontamination processes to which they have been subjected and also to the patient on whom they have been used, thus tracking systems, such as bar codes or RFIDs, are steadily becoming more common. The ability to track and trace surgical instruments and equipment through the decontamination life-cycle enables corrective action to be taken when necessary.
Suggested inspection system configurations:
EVO Cam ll digital microscope: Multi-axis or Ergo stand; LED ring-light and USB for image capturing of defects or failures.
Lynx EVO eyepiece-less stereo microscope: for the inspection of the bar codes used to track and trace the instruments.



